
and compare this to water:
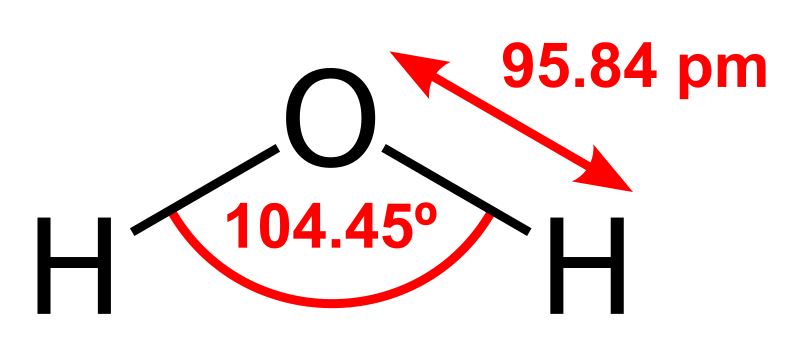
Both molecules are polar, small, and neutral in charge. Once inside a bacteria, algae, or other cell the chlorine atom attached to hypochlorous acid can react with nitrogenous chemicals including some amino acids, proteins, and DNA and in some cases oxidizes or partially oxidizes these chemicals. This disrupts chemical processes in the cell preventing the cell from reproducing.


